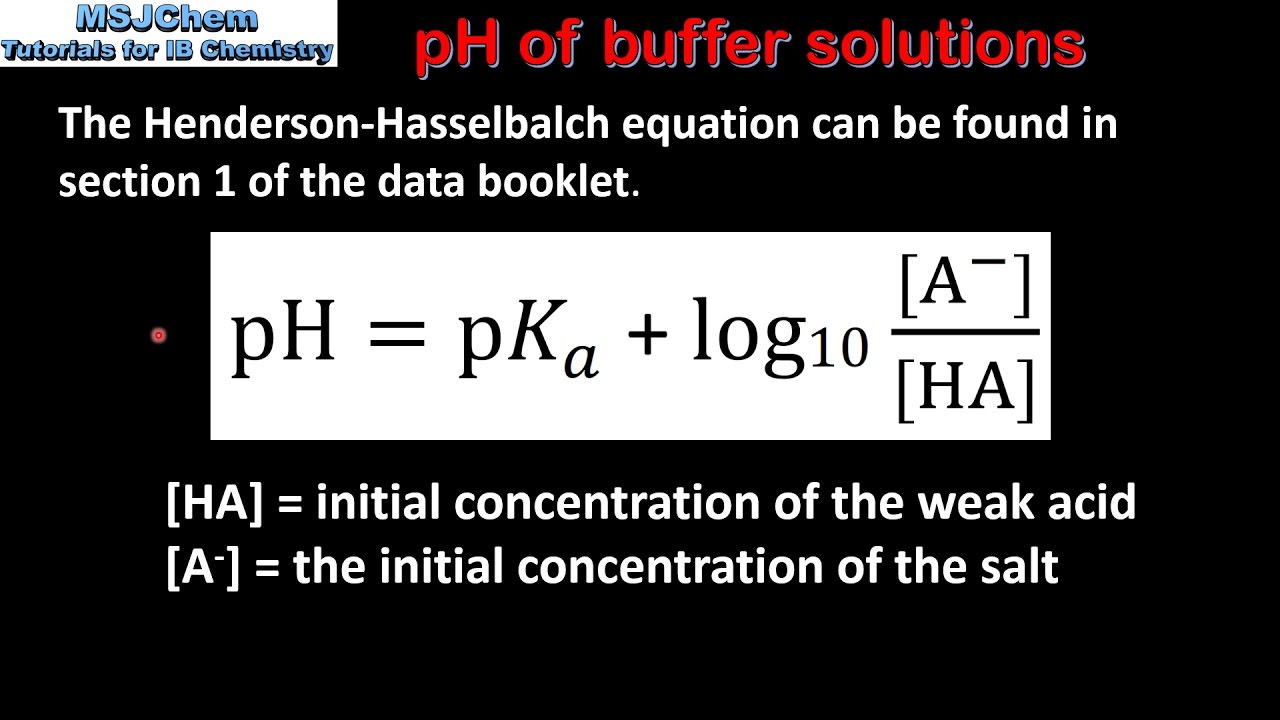
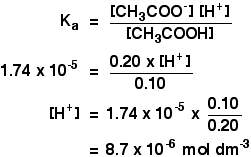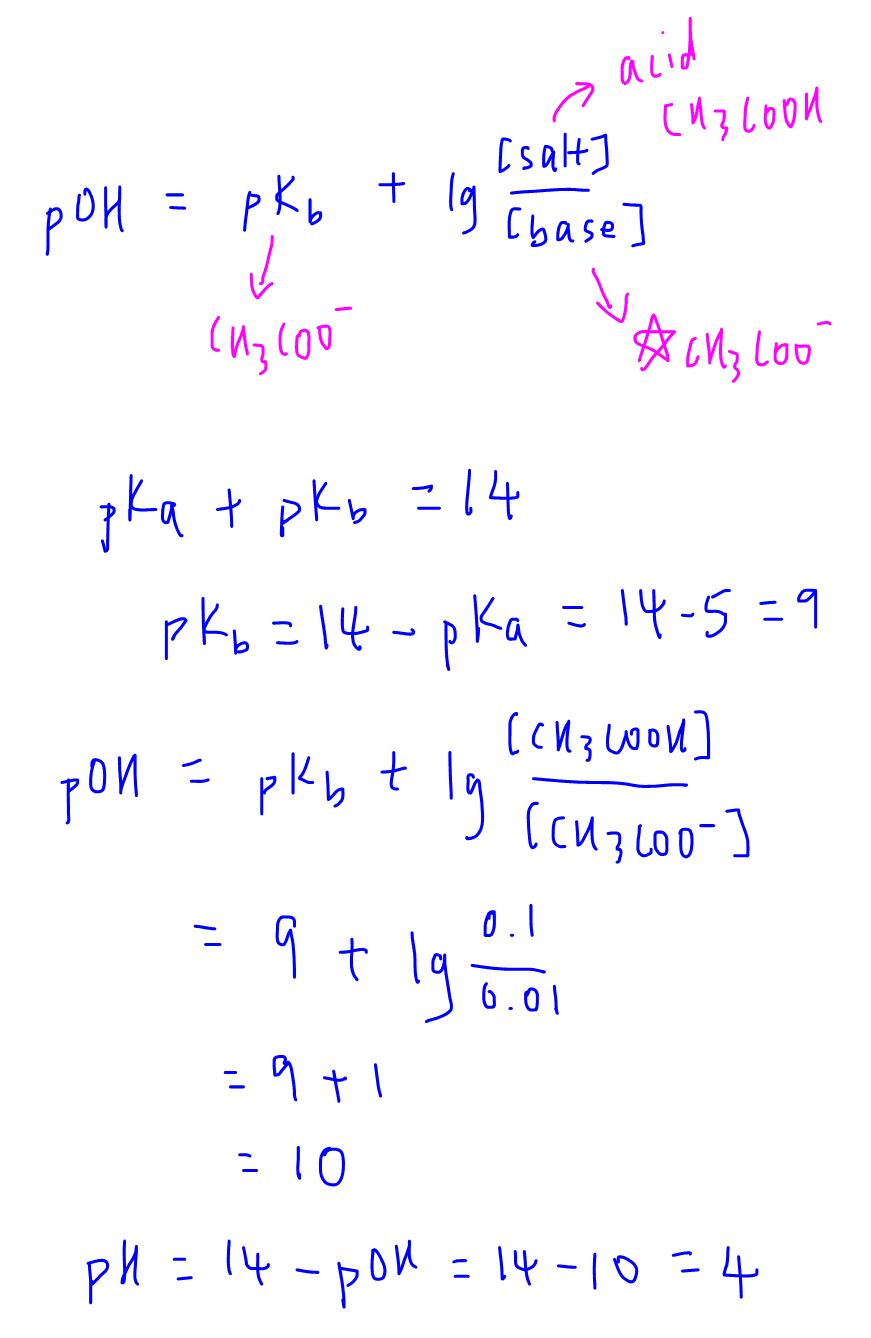![Calculate the pH of buffer solution containing 0.05mol NaF per litre and 0.015 mol HF per litre. [Ka = 7.2 xx 10^(-4) for HF]. Calculate the pH of buffer solution containing 0.05mol NaF per litre and 0.015 mol HF per litre. [Ka = 7.2 xx 10^(-4) for HF].](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/643088122_web.png)
Calculate the pH of buffer solution containing 0.05mol NaF per litre and 0.015 mol HF per litre. [Ka = 7.2 xx 10^(-4) for HF].

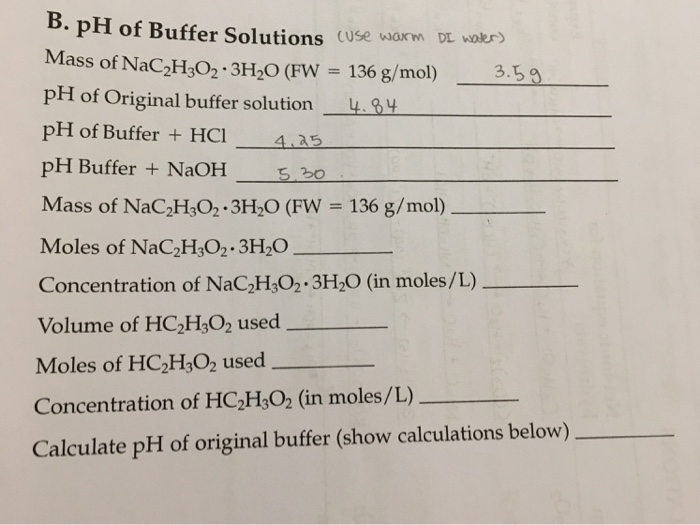
SOLVED: B. pH of Buffer Solutions '9. 50 9 Mass of NaCzH;Oz * 3HzO (FW 1365 g/mol) 41 pH of Original buffer solution of Buffer + HCI 4S pH pH Buffer +
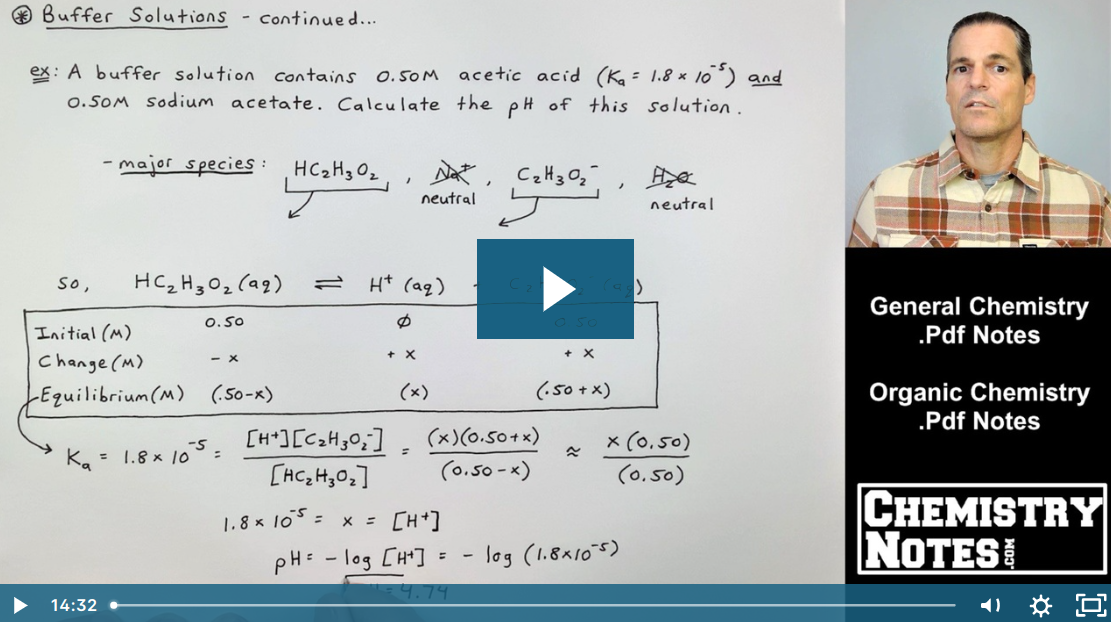
![Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ] Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ]](https://d1hhj0t1vdqi7c.cloudfront.net/v1/dDlCNVZnUE9URzQ=/sd/)
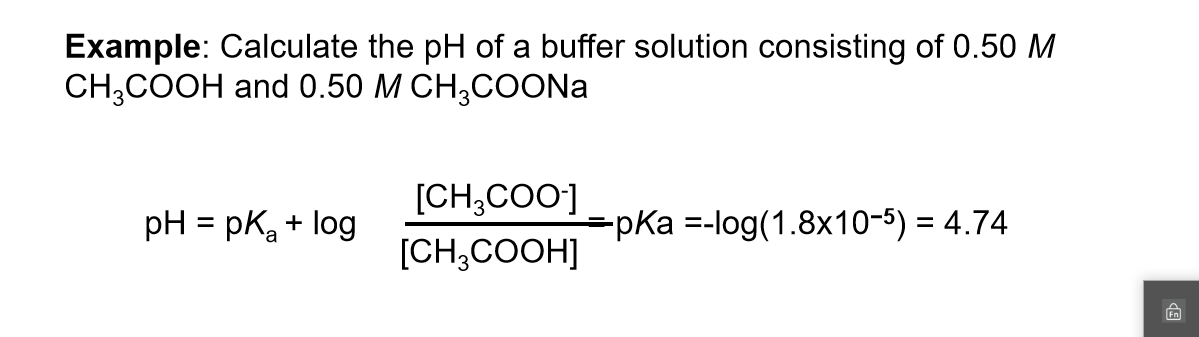
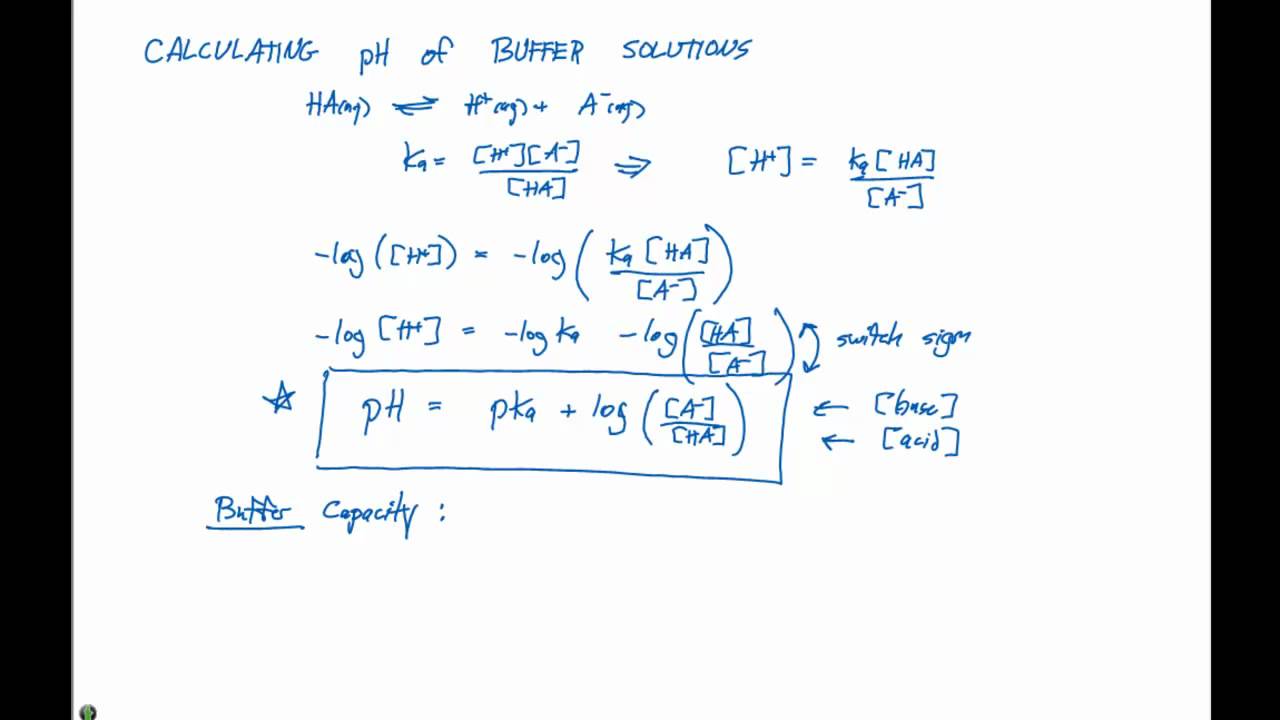
Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ]

PPT – Calculate the pH of the 0.30 M NH3/0.36 M NH4Cl buffer system. What is the pH after the addition of 20.0 mL of 0.050 M NaOH to 80.0 mL of

Calculate the PH of a buffer solution prepared by dissolving 30g of Na2CO3 in 500 ml of an aqueous solution containing 150 ml of 1m HCL . ka for HCO^-3 = 5.63 x 10 - 11