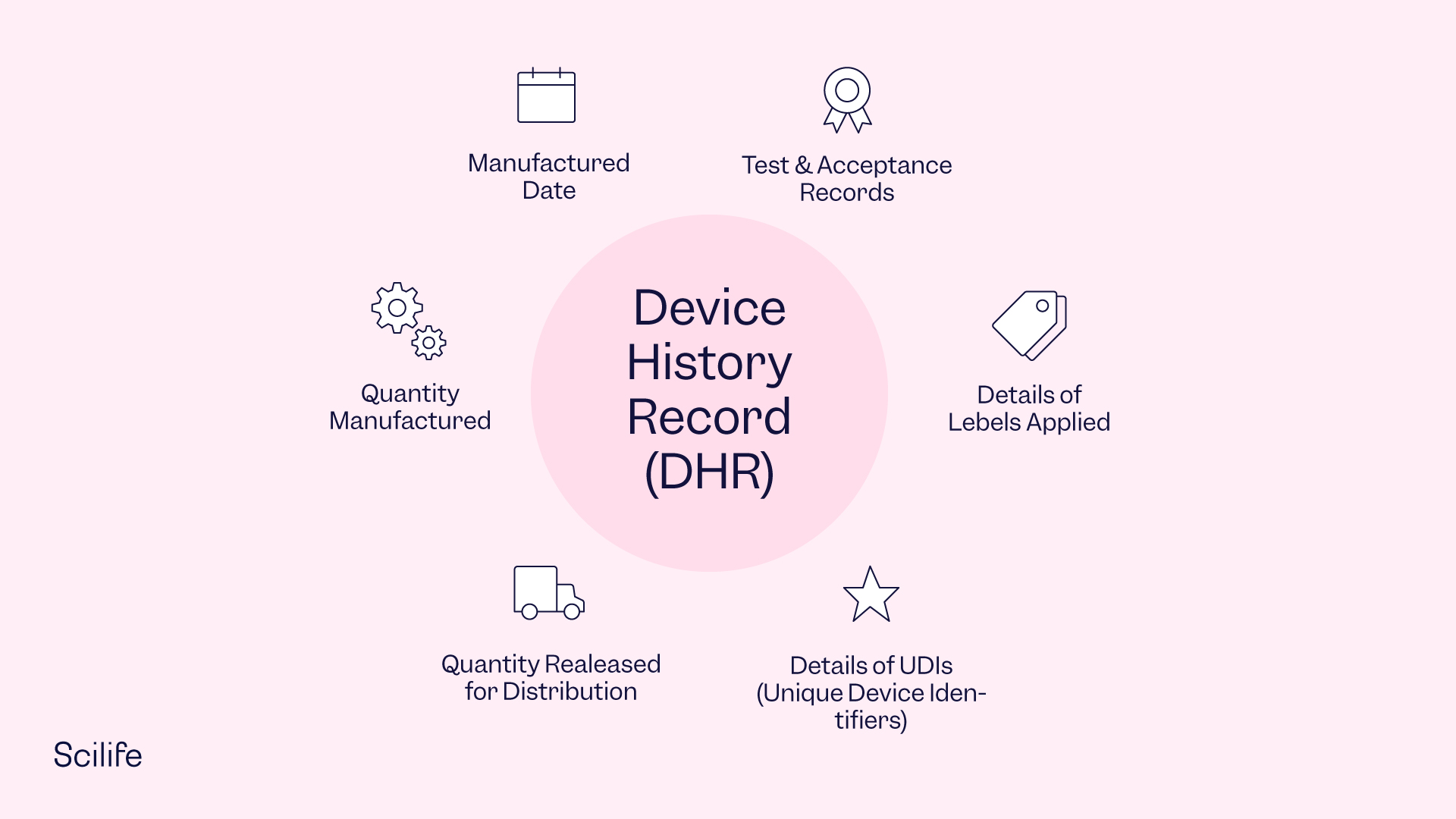
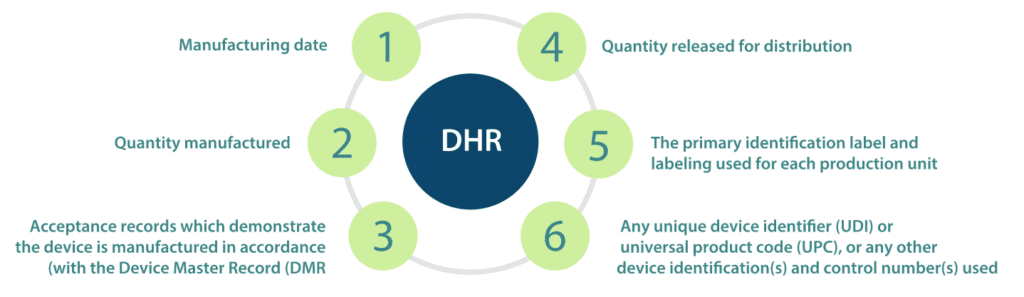
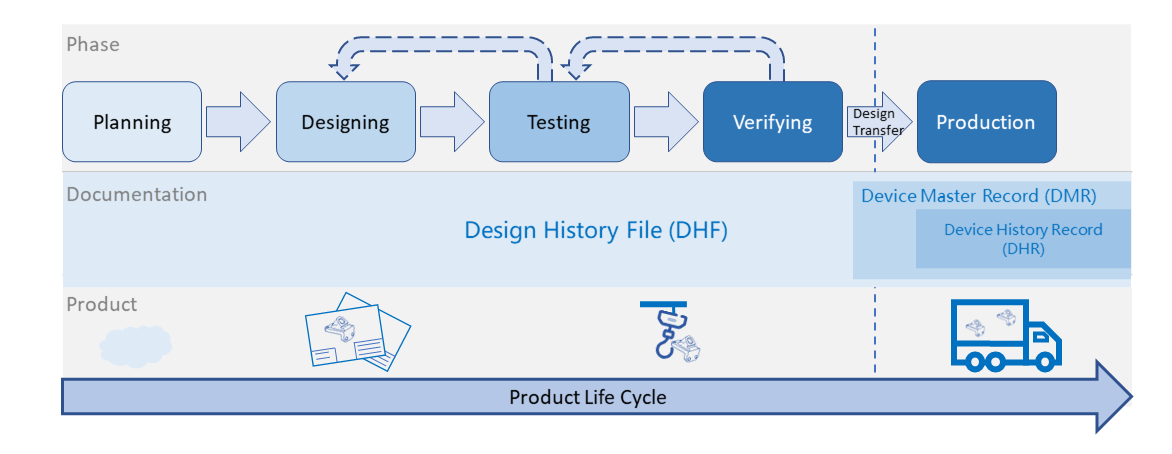
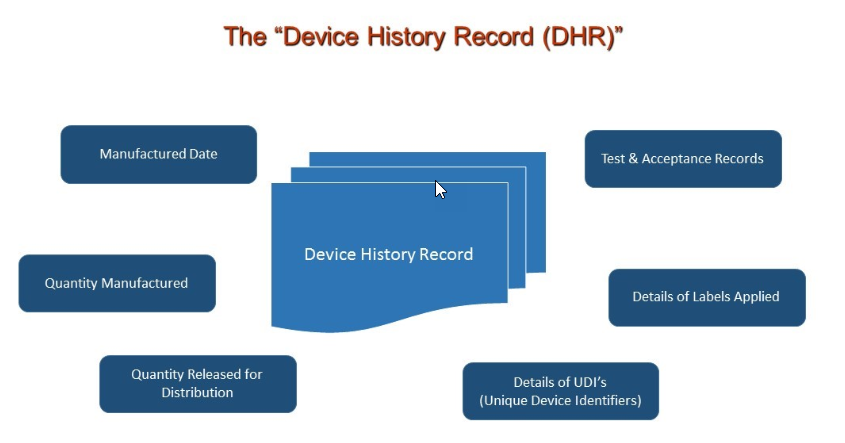
Design History File (DHF) vs. Device Master Record (DMR) vs. Device History Record (DHR): What's the Difference?

Design History File DHF, Device Master Record DMR, Device History Record DHR and Technical File TF - YouTube

Design History File (DHF), the Device Master Record (DMR) and the Device History Record (DHR) - YouTube

Nucleus Consultants på Twitter: "Confused with these terms ? Medical Device File / Device Master Record / Device Master File / Design & Development File / Design History File / Device History
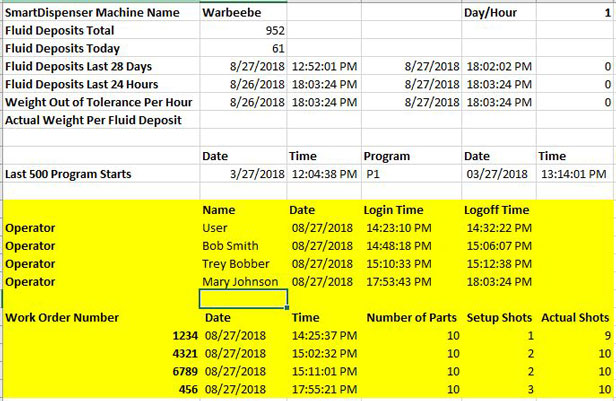
OBSERVATION 1 The device history record does not demonstrate that the device was manufactured in accordance with the device ma