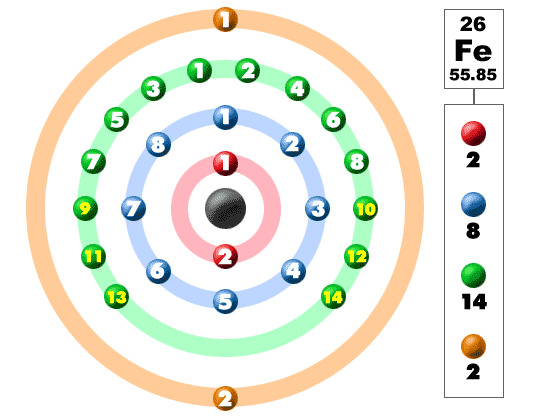
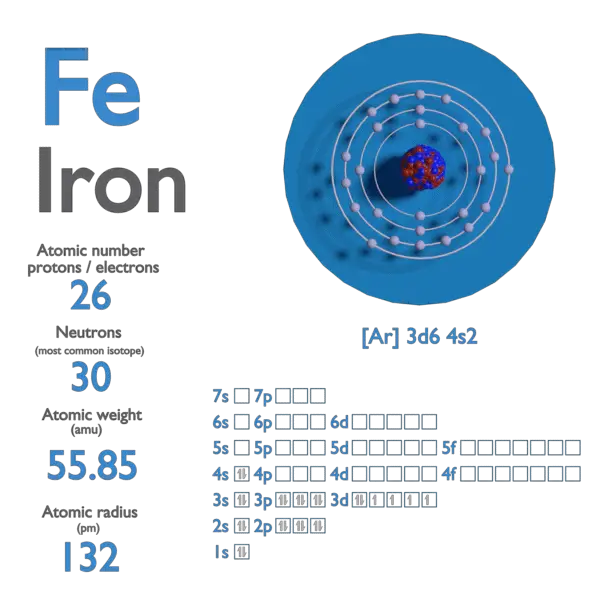
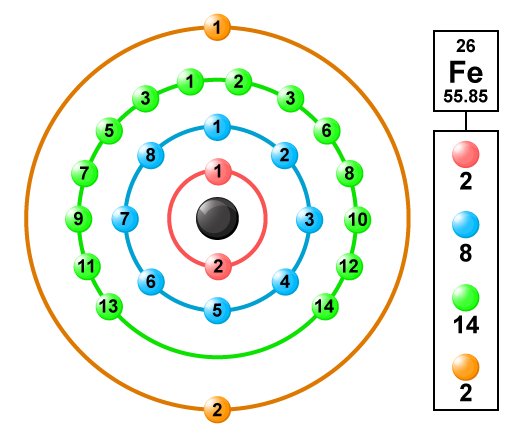
Fe Iron Element Information: Facts, Properties, Trends, Uses and comparison - Periodic Table of the Elements | SchoolMyKids

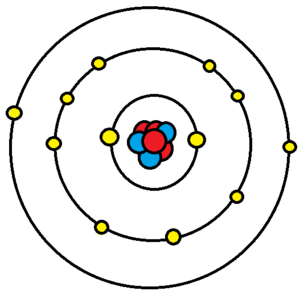
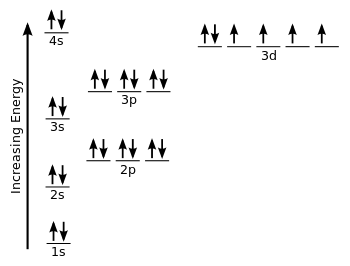
WARM UP Write electron configurations for the following atoms (hint: 2, 8, 8, 18, etc.). Iron (26 e - ) Uranium (92 e - ) Potassium (18 e - ) - ppt download

Fe Iron Element Information: Facts, Properties, Trends, Uses and comparison - Periodic Table of the Elements | SchoolMyKids
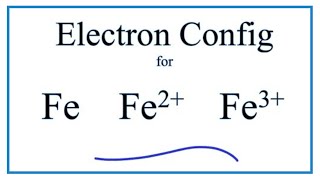
![SOLVED: Write condensed electron configurations for the following atoms and example the electron configuration for Fe is [Ar]4s(2)3d(6). (a) Cr = [Arj3d(4)4s(2) X (b) Ni [Arj3d(8)4s(2) (c) Cu = [Arj4s(1)3d(10) V (d) SOLVED: Write condensed electron configurations for the following atoms and example the electron configuration for Fe is [Ar]4s(2)3d(6). (a) Cr = [Arj3d(4)4s(2) X (b) Ni [Arj3d(8)4s(2) (c) Cu = [Arj4s(1)3d(10) V (d)](https://cdn.numerade.com/ask_images/b77bc7874328406586b14f5eacaa7352.jpg)
SOLVED: Write condensed electron configurations for the following atoms and example the electron configuration for Fe is [Ar]4s(2)3d(6). (a) Cr = [Arj3d(4)4s(2) X (b) Ni [Arj3d(8)4s(2) (c) Cu = [Arj4s(1)3d(10) V (d)