Concentrated `HNO_(3)` is 69% by mass of nitric acid. Calculate the volume of the solution which - YouTube

SOLVED: Given a HNO3 partial pressure in the atmosphere of 4.2 x 10-12 atm, KH = 2.1x105 M/atm, and Ka = 41 M for nitric acid, calculate the equilibrium concentration of nitric

Calculate the concentration of nitric acid in moles per litre in a sample which has density 1.41g/mL - YouTube

Calculate the concentration of nitric acid in moles per litre in a sample which has a density - YouTube

calculate the concentration of nitric acid in moles per litre in a sample which has a density on - Brainly.in

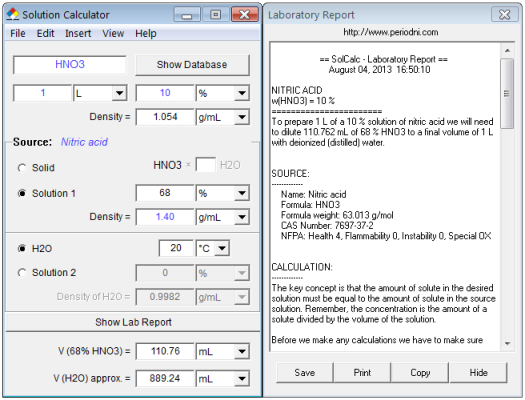
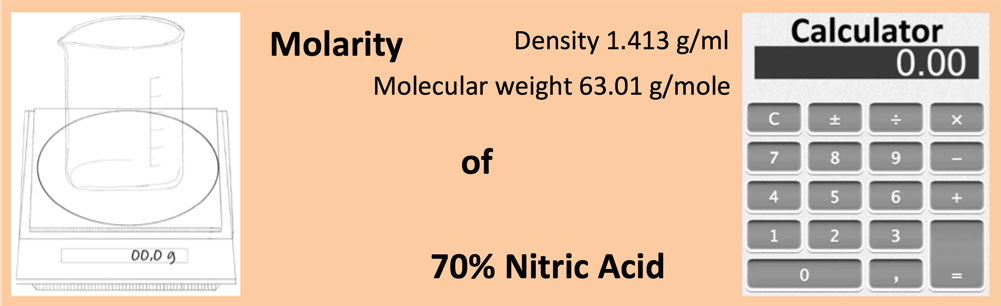
Calculate the concentration of nitric acid in moles per litre in a sample which has a density 1.41 g mL^-1 and the mass per cent of nitric acid in it being 69% .

SOLVED: Enter your answer in the provided box: Nitric acid (HNO;) is used in the production of fertilizer; dyes, drugs; and explosives Calculate the pH of # HNOz solution baving : hydrogen

Calculate the concentration of nitric acid in moles per litre in a sample which has a density 1.41 g //mL and the mass percent of nitric acid in it being 69%.

SOLVED: Calculate the expected pH of a 0.00075 M solution of the strong acid HNO3 Report to 2 decimal places.

Calculate the concentration of nitric acid in moles per litre in a sample which has a density of 1.41g mL^-1 and the mass per cent of nitric acid in it being 69