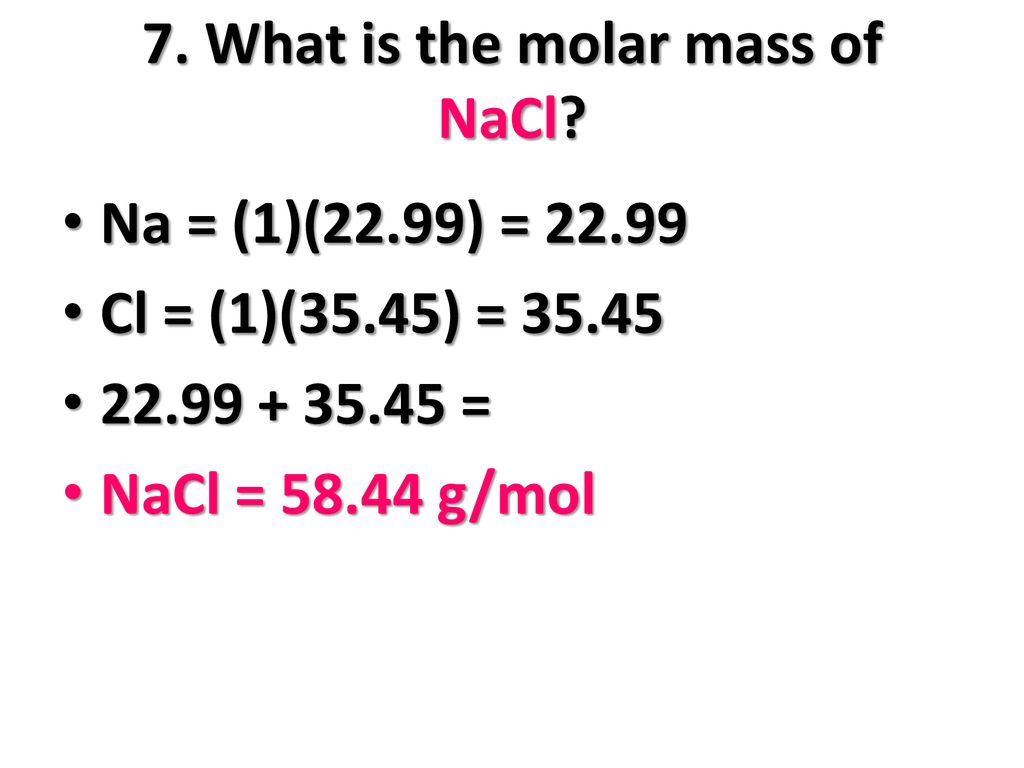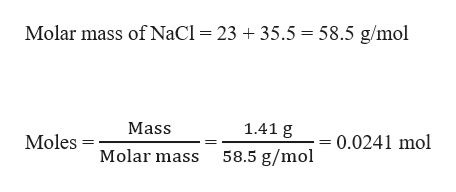![Calculate the number of moles in 50 g of NaCl [Atomic mass of Ca=40 u C=12uO=16 u N=14 u Na=23 u Cl=35.5 u V_{A}=6.022times 10^{23} mol^{-1}] | Snapsolve Calculate the number of moles in 50 g of NaCl [Atomic mass of Ca=40 u C=12uO=16 u N=14 u Na=23 u Cl=35.5 u V_{A}=6.022times 10^{23} mol^{-1}] | Snapsolve](https://wb-qb-sg-oss.bytededu.com/merge/9f7531ed3f3fdcce47dec6f4140c4d32.jpg)
Calculate the number of moles in 50 g of NaCl [Atomic mass of Ca=40 u C=12uO=16 u N=14 u Na=23 u Cl=35.5 u V_{A}=6.022times 10^{23} mol^{-1}] | Snapsolve

Molar Mass. A compound is a collection of bonded together. Ethanol, CH 3 CH 2 OH, is made up of : carbon atoms, hydrogen atoms, and oxygen atom, ppt download

25. The molecular weight of NaCl determined by studying freezing point depression of its 0.5% aqueous solution is 30. The apparent degree of dissociation of NaCl is (1) 0.95 (2) 0.45 (3) 0.60 (4) 0.35

MOLAR MASS Molar mass of a substance = mass in grams of one mole of the substance. A compound's molar mass is NUMERICALLY equal to its formula mass. Formula. - ppt download

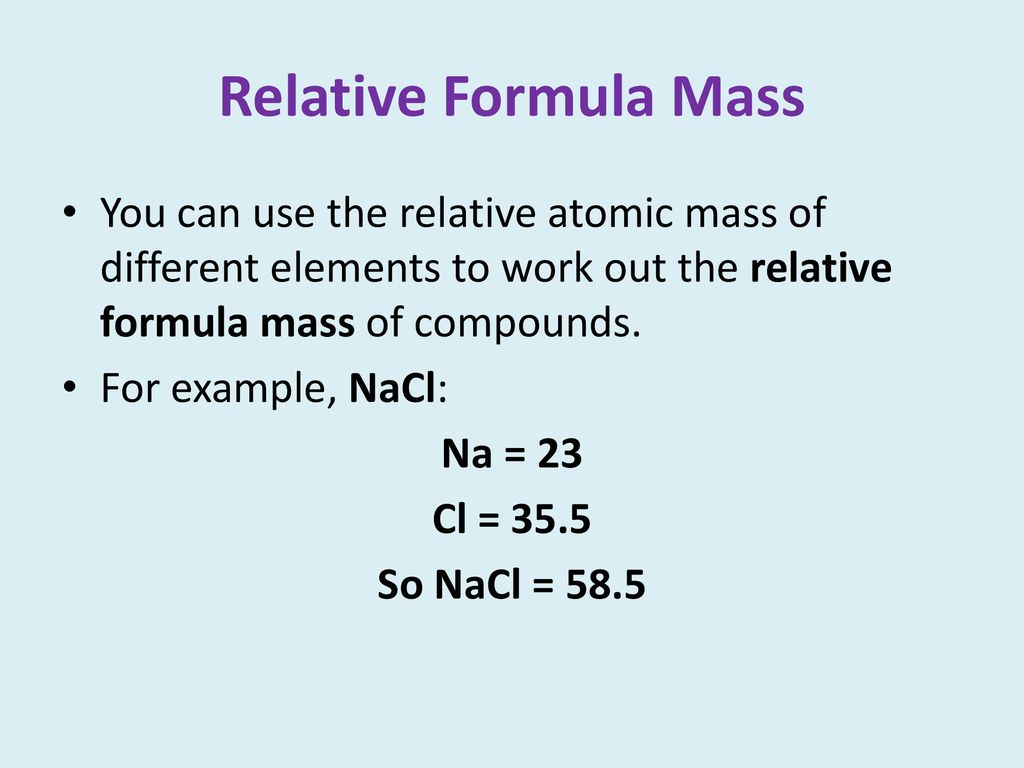
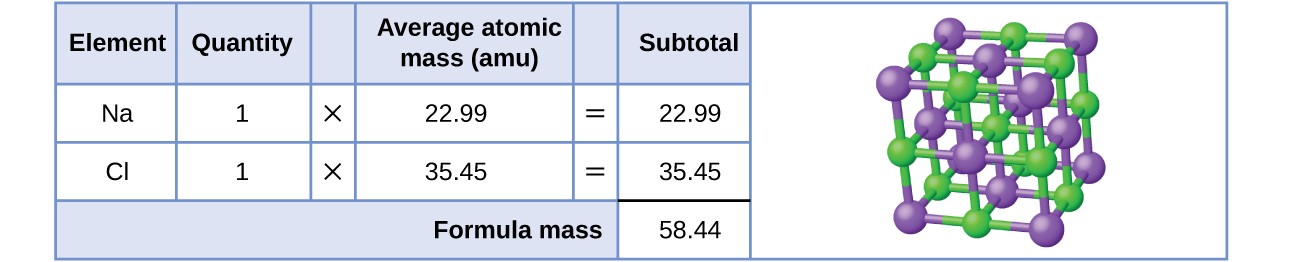
1 Formula mass is the sum of the atomic masses (in amu) in a formula unit of an ionic compound. 1Na22.99 amu 1Cl amu NaCl amu For any ionic. - ppt download
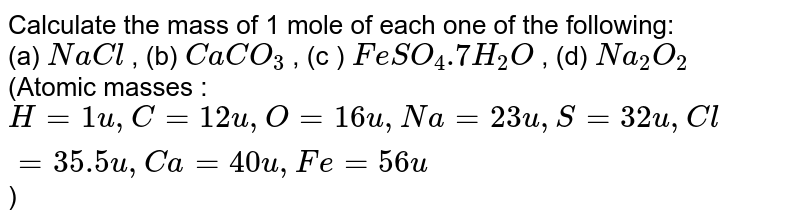
Calculate the mass of 1 mole of each one of the following: (a) NaCl , (b) CaCO(3) , (c ) FeSO(4).7H(2)O

The atomic masses of sodium and chlorine are 23 and 35.5 respectively . What is the formula mass of sodium chl

The reaction, 2A(g) + B(g) 3C(g) + D(g) , is begun with concentration of A and B both at initial value of 1 M . When equilibrium is reached, the concentration of
Formula mass of NaCl is 58.45 g mol^-1 and density of its pure form is 2.167 g cm^-3. The average distance - Sarthaks eConnect | Largest Online Education Community